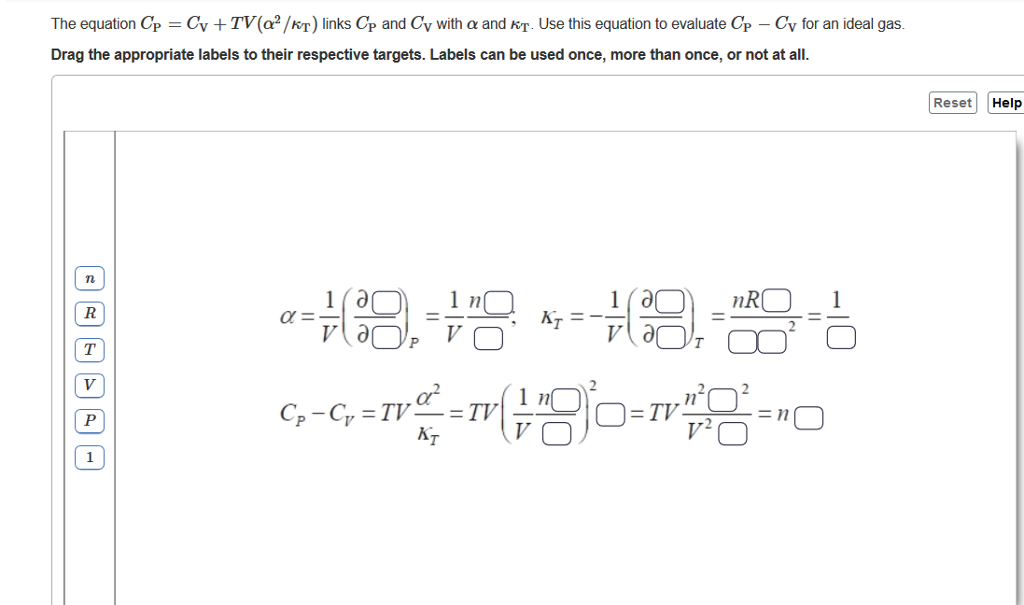
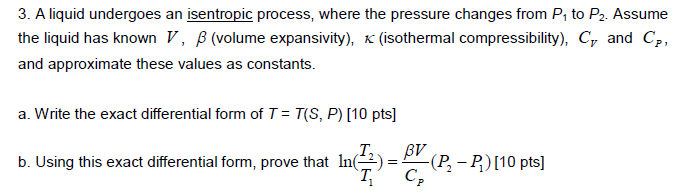
If CP and CV denote the specific heats of nitrogen per unit mass at constant pressure and constant volume respectively, then.

OpenFOAM-9 multiphaseEulerFoam, wallBoiling, changing table Hs to accommodate new pressure : r/OpenFOAM

Cp-Cv for real gas, in terms of alpha & beta, change in internal energy with respect to volume - YouTube

Cp/Cv=gamma Proof | Relation Between Specific Heats & Adiabatic Index | Properties of Gases | BME - YouTube

The Circle of Omicron Delta Kappa, Annual Report 2022, Vol. 101, No.1 by Associate Executive Director - Issuu
Ideal and Real Discharge Coefficients – Using Fundamental Equations of State in Mass-Flow Measurements with Sonic Nozzles R. S

Development in the Concept of Bacterial Polysaccharide Repeating Unit-Based Antibacterial Conjugate Vaccines | ACS Infectious Diseases

Energy Conversion CHE 450/550. Ideal Gas Basics and Heat Capacities - I Ideal gas: – a theoretical gas composed of a set of non-interacting point particles. - ppt download